Projects
Molecular Biology of Signalling in Inflammatory Processes
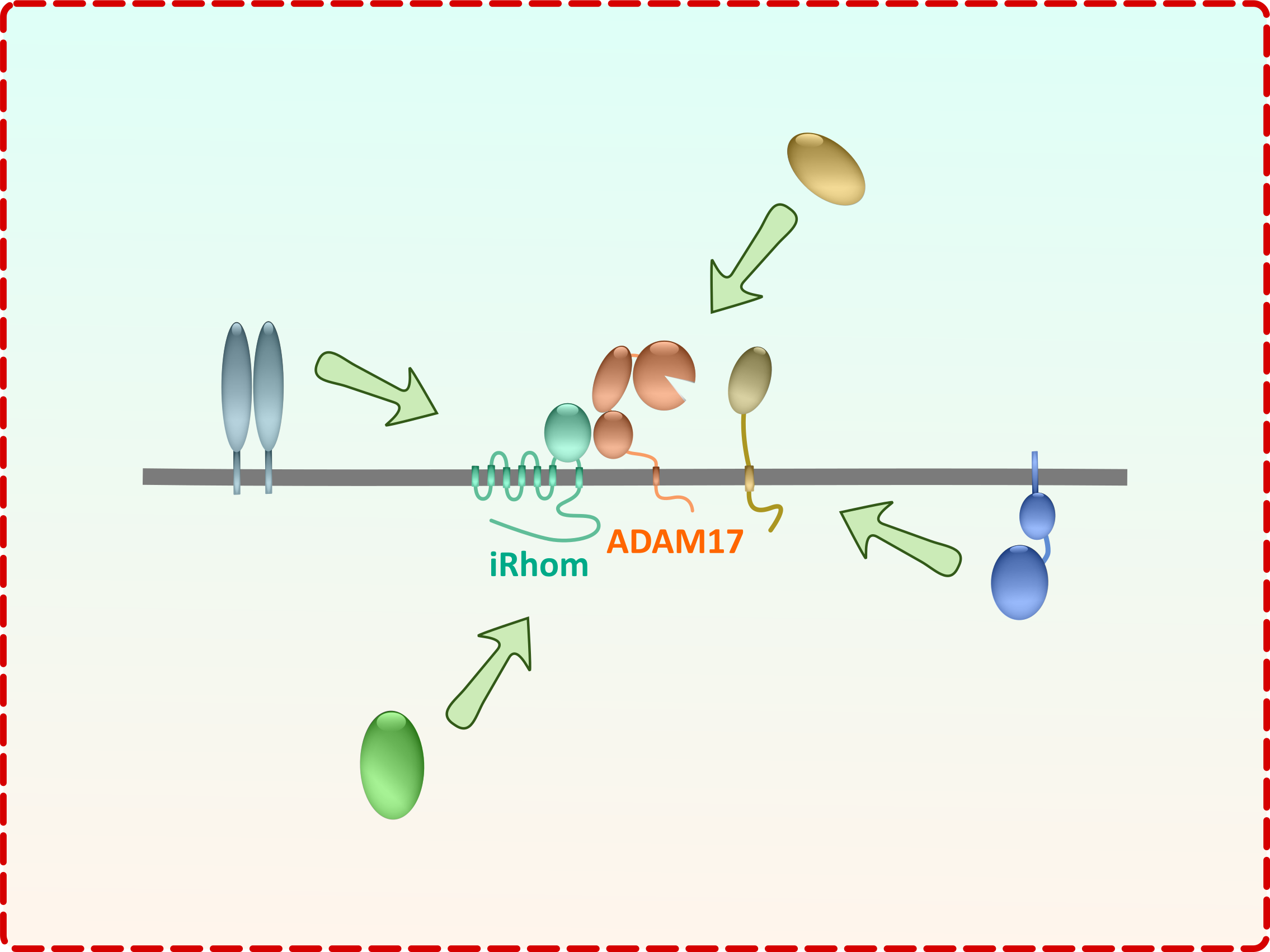
The iRhom-ADAM17 complex
To understand the regulation of the shedding complex consisting of iRhom-ADAM17 at its core, we first try to establish the structure-function relationships of both proteins (Projects A and B). The next consequential step is the identification and analysis of additional permanent as well as transient binding partners of this dynamic complex (Project C). This will also support our efforts to identify the as yet unknown molecular mechanism of the forward trafficking and localisation of this complex (Project D).
The results of this project will help us to explore the regulation and consequences of the iRhom-ADAM17 complex on (patho)pysiological processes in ex vivo and in vivo settings. (Project E).
Furthermore, since ADAM17 shares many substrates with other proteases, we also analyse the protease network of shared substrates involved in a signalling network (Project F).
Reviews
[1] Düsterhöft, S., A. Babendreyer, A. A. Giese, C. Flasshove and A. Ludwig (2019). "Status update on iRhom and ADAM17: It's still complicated." Biochim Biophys Acta Mol Cell Res 1866(10): 1567-1583. DOI: 10.1016/j.bbamcr.2019.06.017
[2] Düsterhöft, S., J. Lokau and C. Garbers (2019). "The metalloprotease ADAM17 in inflammation and cancer." Pathol Res Pract 215(6): 152410. DOI: 10.1016/j.prp.2019.04.002
[3] Grötzinger, J., I. Lorenzen and S. Düsterhöft (2017). "Molecular insights into the multilayered regulation of ADAM17: The role of the extracellular region." Biochim Biophys Acta Mol Cell Res 1864(11 Pt B): 2088-2095. DOI: 10.1016/j.bbamcr.2017.05.024
[4] Düsterhöft, S., U. Künzel and M. Freeman (2017). "Rhomboid proteases in human disease: Mechanisms and future prospects." Biochim Biophys Acta Mol Cell Res 1864(11 Pt B): 2200-2209. DOI: 10.1016/j.bbamcr.2017.04.016